Abstract
Boron nitride nanotubes (BNNTs) will be one of the most important materials of this century. Recent synthetic advances have made BNNTs viable candidates for advanced multifunctional materials. Like carbon nanotubes and graphene, BNNTs and h-BN have extraordinary physical properties. Unlike CNTs, BNNTs have a wideband gap; they are piezoelectric, have neutron radiation shielding capability, and can withstand degradation up to 1000 °C. BNNTs could be the next big leap for nanocomposite advanced applications; however, significant scientific challenges must be addressed. Predominantly, large-scale synthesis techniques are immature. Production products require careful characterization, analysis, and purification. Impurities such as boron, amorphous BN, and h-BN lead to difficulty studying chemical modification and translation of BNNT properties. This review synthesizes relevant literature and state-of-the-art techniques regarding purification methods of BNNTs, classified as physical, chemical, and multi-step techniques and their applications. The review also discusses BNNT synthesis methods and future research directions.
Graphical abstract

Similar content being viewed by others
Introduction
Attention to boron nitride nanotubes (BNNTs) and related research have seen a tremendous increase recently due to emerging commercial BNNTs availability and their excellent physical and chemical properties. Boron nitride (BN) is isoelectronic and isostructural to carbon and will form analogous allotropes to carbon nanotubes and graphene. BNNTs are the carbon nanotube BN analog, a one-dimensional high aspect ratio nanostructure of hexagonal boron nitride (h-BN), where the boron and nitrogen atoms alternately substitute the carbon atoms. Like multi-walled carbon nanotubes in structure, BNNTs possess a multi-walled nanometer diameter in the range of 1–100 nm and can be micrometers in length [1]. Depending on the radius of the tube and chirality, carbon nanotubes (CNTs) can be conductors or semiconductors, while chirality in BNNTs affects the piezoelectric actuation vector in the tube [2]. Conversely, BNNTs are always insulating with a wide bandgap of ~ 5 to 6 eV [3] regardless of tube morphology and chirality (Table 1). Besides the bright white appearance due to their large bandgap, BNNTs and h-BN platelets notably have a high resistance to oxidation up to 900 °C [4] in air and higher thermal and chemical stability in inert high-temperature environments.
BNNTs are isoelectronic with carbon but possess different chemical properties compared to CNTs because of their partial ionic bonding resulting from the difference in electronegativity of nitrogen and boron atoms [7]. Because of their large bandgap, BNNTs are electrical insulators; however, they maintain high thermal conductivity [8] similar to graphitic nanoparticles. They also have high hydrophobicity [9], high neutron absorption ability [10], transparency in the visible and infrared region of the electromagnetic spectrum [11, 12], are piezoelectric [13, 14], and have a high Young’s and shear modulus (1.8 ± 0.3 TPa and 7 ± 1 GPa, respectively) [15, 16]. The wide array of remarkable properties of boron nitride nanoparticles positions them as important advanced materials for future technology development encompassing many disciplines. Due to the possible non-cytotoxic nature of BNNTs, they are also useful for diagnostic and therapeutic biomedical applications [17, 18].
The use of BNNTs in various applications is highly dependent on the synthesis method and resulting purity. Various techniques being used to synthesize BNNTs include laser ablation, arc discharge, autoclave, chemical vapor deposition, ball milling, etc. Most techniques produce powder containing BNNTs and impurities, such as hexagonal BN, BN nanoparticles, the remnant of different precursor materials used, and other compounds of boron and nitrogen which are hard to remove. Some of the techniques like catalytic chemical vapor deposition [19] and ball milling [20] use a metallic catalyst that remains with the produced BNNTs. Removing some BNNT impurities requires aggressive treatments, which will cause damage to the yield and structure of the BNNTs. This damage hinders the ideal performance of BNNTs in new applications and complicates the analysis of the BNNT's characteristics.
The presence of impurities in as-synthesized BNNTs limits use and the development of innovative applications that depend on BNNT purity. The impurities, such as h-BN, amorphous boron, amorphous BN, and metal catalyst contained in the as-synthesized BNNTs reduce the chemical and physical properties of the bulk material. Impurities reduce the benefit of BNNTs in applications requiring pristine-structured nanotubes or aligned arrangements of nanotubes and dramatically increase the cost of BNNT composites. For example, applications harnessing the thermal conductivity of BNNTs require the alignment of the tubes in the direction of heat movement [21]. Aligned BNNTs are being considered for fillers to fabricate composites [22] that act as thermal interface material between a heat source and heat sink in energy and microelectronic applications such as making batteries [23], and printed circuit boards [24]. For biological applications like drug delivery that require tubes with tips opened for access to the central canal, metal impurities at the tip of the BNNT must be removed. Impurities reduce our ability to design encapsulation motifs and complicate drug encapsulation and increase the cytotoxicity of BNNTs in the cell [25, 26].
To fully harness the unique properties of BNNTs, effective methods of purification which remove impurities and retain the original length and structure of BNNT are needed. Various purification methods have been reported in works of literature. BNNT purification processes will typically combine several methods, depending on the synthesis process, nature of impurities, nanotube morphologies, and intended applications. In general, purification is categorized as a physical or chemical method. Filtration, surfactant, and polymer wrapping are physical methods that have been used to separate impurities from BN nanotubes based on their length, size, and solubility [27]. These methods are generally used to remove aggregate and amorphous compounds of boron and BN. Often, physical methods are considered less effective compared to the chemical methods; however, they are relatively mild and do not damage or modify the tubes [28].
Chemical methods such as thermal etching and oxidation by acids separate the impurities from BNNTs based on their reactivity. These harsh processes have a high potential for causing product loss, BNNT morphology damage, introducing defects along the tubes, or causing chemical modification of the tubes, depending on the exact conditions used during the process. [29]. More recently, it has been shown that appropriate control of reaction conditions can produce high-purity BNNTs with opened tips [30].
This review curates the state-of-the-art BNNT purification literature and organizes the various techniques being used to purify BNNTs. Herein, purification is discussed in the context of the BNNT synthesis method, the purification results, and changes to the structure or morphology of the BNNTs obtained with each method. The type and quantity of impurities present in as-synthesized BNNT depend on the boron and nitrogen source, synthesis method, and type of catalyst used. This review highlights the physical and chemical methods of purification used in addressing the various type of impurities and discusses and compares the mechanism and effectiveness of each purification technique.
Synthesis and impurities of BNNTS
BNNT synthesis techniques can be classified as high-temperature, above 2000 °C, or low-temperature methods, below 2000 °C. High-temperature techniques include arc-discharge, plasma-enhanced systems, and laser ablation methods, which involve vaporizing boron element or boron nitride (vaporization temperature of boron depending on pressure is over 4000 °C). Ball milling, template synthesis, and chemical deposition methods operate at temperatures between 600 and 1700 °C. One of the essential factors considered when selecting the BNNT synthesis method is the conversion rate of nitrogen and boron source to BN radical [31]. The size and length of BNNTs produced are dependent on the synthesis method, boron precursor, nitrogen source, temperature, catalyst, equipment, duration, and mode of heating.
Arc-discharge
The first successful synthesis of BNNTs was demonstrated by Alexander Zettl’s group at UC Berkeley in 1995 and published in Chopra et al. [32]. Synthesis involves the use of a carbon-free plasma discharge between a copper electrode and a BN tungsten rod cathode to produce multi-walled BNNTs at an operating temperature of ~ 3500 °C. Further research on the arc-discharge method led to the production of single-walled boron nitride nanotubes (SWBNNTs) and multi-walled boron nitride nanotubes (MWBNNTs). The produced nanotubes are highly crystalline but contain BN onion and cage-like impurities. Variations of the original arc-discharge experiment have involved compounds of boron such as Yb6, HfB2, and ZrB2, which are conductive, and were used as a cathode electrode in the nitrogen atmosphere of the arc-discharge method [33, 34]. Yeh et al. [35] also successfully used a boron anode and boron/tungsten cathode for the synthesis of BNNTs through arc discharge. The BNNTs synthesized were mainly single and double walled and were found on all surfaces in the chamber without a specific deposition location. The final produced BNNTs contained impurities such as unreacted boron anode material, wrapped BN layers, unreacted cobalt/ nickel, and h-BN.
Laser ablation
Laser ablation is a high-temperature, high-pressure process that involves vaporizing a boron or BN source target with continuous CO2 laser in a nitrogen/inert gas-filled chamber (Fig. 1) [36]. The nanotubes are deposited on the cooler surfaces of the reactor as the vaporized boron nucleate and grow. Collectors are installed at different heights/positions in the system to collect the nanotubes [37].
Schematic of BNNT synthesis in the HTP laser ablation process. Adapted with permission from [37].
Golberg et al. introduced the laser ablation method for BNNTs synthesis [38]. He produced MWBNNTs by heating a crystal of cubic BN to 5000 K at high nitrogen pressure (5–15 GPa) using a CO2 laser. Subsequent research has used a hexagonal BN target [39] as a precursor to producing highly crystalline BNNTs with few defects and small numbers of walls, without the use of catalyst [40]. Impurities such as amorphous boron flakes, BN cones, and BN onions were found in the final BNNT product.
Kim et al. [37] also used boron fibers with a diameter of 102 µm and nitrogen gas as precursors in a high-temperature pressure (HTP) laser ablation system for BNNT synthesis. They heated the boron fiber continuously with a 1000 W CO2 laser in nitrogen gas, with the temperature of the molten ball formed at the point of irradiation reaching up to 5101 K. The cotton-like BNNTs produced were collected at a specific height in the chamber above the produced molten boron ball (see Fig. 1) and contained other boron nitride nanostructures like h-BN and boron nanoparticles as impurities.
Plasma-enhanced systems
In general, plasma-enhanced systems use extremely high-temperature plasma (exceeding several thousand degrees) to vaporize boron and nitrogen precursors. After passing through the high-temperature plasma region, the highly concentrated boron vapor nucleates to form boron nanoparticles. The boron nanoparticles formed during the plasma process serve as the seed material for the growth of the BNNTs [41].
Kim et al. [11] created BNNTs in an induction thermal plasma reactor by introducing solid hexagonal boron nitride (h-BN) powder, nitrogen, and hydrogen gases as precursors, into a high-temperature plasma (> 8000 °C) at atmospheric pressure. These precursor materials were immediately vaporized within the plasma, breaking them down into their constituent elements; B, N, and H. Outside the plasma, the boron vapor condenses into nanodroplets that initiate BNNT formation, with hydrogen gas acting as a catalyst. This process generated highly crystalline BNNTs with a 20 g/h production rate and an average diameter of 5 nm. The synthesized BNNTs were extracted from the plasma reactor as macroscopic sheets, yarns, bucky papers, and transparent thin films [11]. Fathalizadeh et al. [42] designed and custom-built an extended pressure inductively coupled plasma system (EPIC) (Fig. 2) for the synthesis of high-quality BNNTs. The EPIC system allows direct and continuous injection of precursor material (liquid, gas, or solid) into its variable-power plasma plume. The process produces BNNTs with a high aspect ratio at a production rate of 35 g/h and contains unreacted boron nanoscale particles as impurities [42].
Schematics of extended pressure inductively coupled plasma system (EPIC). Ports A, B, and C are for injection of precursor material and plasma gas. Ports D–K are for system diagnostics and/or pressure-assisted purging of material. Adapted with permission from [42].
Notably, these high-temperature synthesis methods, such as plasma-enhanced and pressurized vapor/condenser methods are making great advances in synthesizing high-purity BNNTs at industrially relevant amounts [36, 42]. However, purity is still a challenge.
Ball milling
Ball milling is a two-step mechano-thermal process employed to produce BNNTs. It involves the ball milling of the powder precursors to produce amorphous or defective structure and thermal annealing under N2 flow at an operating temperature range of 1000–1300 °C [20]. The mechanical energy created during this process is enough to induce defects and is determined by variables such as intensity (round/minutes) and milling time (which can extend from hours to days). Chen et al. produced the first BNNTs synthesized by ball milling [43]. Chen ball-milled boron powder at room temperature in an NH3 atmosphere for 150 h producing a mixture of nanocrystalline B and disordered BN. Subsequently, the powders were heated at 1200 °C in N2 gas, converting them to BNNTs. The BNNTs produced had a bamboo-like structure and contained bulk BN flakes and amorphous B particle impurities that are difficult to remove due to their stability. Ball milling has also been used to produce BNNTs from B2O3 powder [44] and h-BN powder [45]. To improve the yield of BNNTs, Li et al. [20] milled boron powder with iron nitrate which acts as an effective catalyst, improving the nitriding reaction of boron powder. Magnesium nitrate and Cobalt nitrate can also be used [46]. Ball milling will often produce metal-containing impurities from the mechanical wear of the ball or the metal-containing catalyst used during the process [47].
Template synthesis
Template synthesis is a low-temperature technique used to produce BNNTs that involves the substitution of atoms of the template in a nitrogen or ammonia atmosphere, which acts as a nitrogen source and as a protection. The most commonly used BNNT synthesis templates are carbon nanotubes (CNTs) [48,49,50] due to their structural and isoelectronic similarity to BNNTs. CNTs were reacted with B2O3 in an N2 atmosphere at 1773 K by Golberg et al. [51] to produce multi-walled BNNTs of 2–6 walls. Tay et al. [52] reported the use of CNTs to synthesize vertically aligned BNNTs with lengths reaching over millimeters by using template-assisted chemical vapor deposition at 900 °C. Also, Borowiak-Palen et al. [53] synthesized multi-walled BNNTs by heating CNTs with B2O3 and MoO3 as the catalyst in a nitrogen atmosphere at a temperature of 1500 °C and vacuum pressure of 1027 mbar. The BNNTs produced using template synthesis have controlled morphology but introduce carbon as an impurity that is integrally embedded in the BNNTs due to incomplete substitution during synthesis, which is extremely difficult to remove. Alternatively, this method can also be used to produce B- and N-doped CNTs [54].
Aside from CNTs, another form of the template synthesis carried out by Bechelany et al. [55] is the membrane-assisted template method which involved the use of nanoporous aluminum oxide. Nanoporous aluminum oxide membranes contain an array of well-defined nanoscale cylindrical pores. This study demonstrates that aluminum oxide membranes can serve as an inorganic template for BNNT synthesis. In this study, they infiltrated the alumina membrane template with liquid polymeric borazine. The synthesized borazine underwent thermolysis at 1200 °C. The result is the production of ordered BNNTs joined by the large boron nitride sheet which grew on the surface of the alumina membrane.
Chemical vapor deposition (CVD)
CVD is the most widely known and practiced technique used for the synthesis of CNTs and BNNTs at gram-scale quantities. The CVD technique provides versatile control over growth parameters and reaction conditions when compared with other techniques. This flexibility has led to the wide adoption of CVD BNNT synthesis in smaller industry synthesis settings and academic laboratories and has led to the synthesis of high-quality BNNTs. Based on the variation of precursor, experimental setup, catalyst, growth mechanism, and temperature, various categories of CVD techniques have been established [56]. These methods can be classified into catalytic and non-catalytic CVD.
Catalytic CVD
Various researchers have tested the efficiency of different catalysts (such as Ni2B, MgO, Co, Fe) with varying precursors, by the quality and quantity of BNNT produced. Lourie et al. [57] used borazine (B3N3H6) produced from the reaction of NaBH4 and (NH4)2SO4, as the precursor with NiB metal catalyst at a temperature range of 1000–1100 °C. For this synthesis, the root growth mechanism was evident. The general setup involved the borazine-carrying gas flowing into the furnace containing the NiB catalyst on a silicon wafer substrate. The borazine decomposes into BN and H2 and BNNTs precipitate out of the catalyst particle due to the supersaturation of the BN aggregate. The BNNTs produced were mostly hollow and crystalline multi-walled tubes with flag-like morphology and length of about 5 μm [57]. Chatterjee et al. [19] also used borazine and decaborane as a precursor with floating nickel as a catalyst.
Variations in the catalytic CVD process have introduced precursors such as boron and metal oxides heated with an induction furnace to avoid the use of toxic precursors [58]. Further development resulted in the replacement of the induction furnace with a horizontal tube furnace [59]. The reaction of boron and metal oxides produces boron oxide which reacts with the nitrogen source to form the BNNTs. This technique is referred to as Boron Oxide Chemical Vapor Deposition (BOCVD). Tang et al. [58, 60, 61] used boron as a precursor in a different experiment, varying the precursor’s ratio, catalyst, and environment (nitrogen source). Tang et al. [58] used nanoscale iron oxide (Fe2O3) powder as a catalyst which was heated with boron in a weight ratio of 1:15, in an alumina tube with argon gas flow. Ammonia gas as a nitrogen source was introduced into the system when the operating temperature reached 1200–1500 °C. The BNNTs produced had a variable and discontinuous structure with a diameter range of 7–20 nm. However, the efficiency of the chemical reaction between B and FeO at higher temperatures to produce B2O2 was not sufficient to produce significant BNNTs.
Additionally, Tang et al. [60] synthesized BNNTs at a temperature of 1100–1300 °C in the presence of ammonia or nitrogen gas by heating alumina-supported nanoscale nickel boride catalyst and boron. The BNNTs produced have a length of several microns and a diameter range of 5–40 nm with a crystallized tube structure. At temperatures below 1100 °C and above 1500 °C, there was no formation of BNNTs. At a temperature above 1300 °C, BNNTs with defects in the structure were produced [60]. Subsequently, Tang et al. [61] used magnesium oxide as a catalyst, with equal ratios of magnesium oxide powder and boron heated in the presence of ammonia gas at 1300 °C. This procedure produced pure BNNTs of length 10 µm and diameter up to 70 nm. Defects were found in nanotubes with larger diameters, while the smaller diameter nanotubes had a concentric tubular structure with no defects. This synthesis process had a slow rate of BNNT growth resulting from the slow catalytic activity of the magnesium, which slowed the growth rate of BNNTs. To combat this effect, the authors increased the temperature of the reaction. However, this resulted in the formation of additional impurities such as bulk BN flakes and Mg2B2O5 leading to as low production of BNNTs.
Alternatively, Zhi et al. [62] used a mixture of MgO and FeO as a catalyst for the synthesis of BNNTs. Their goal was to overcome the shortcomings of using each catalyst alone. In these reactions, B2O2 an intermediate product, is converted to BNNTs. With FeO as catalyst, the reaction of B to produce B2O2 was inefficient to supply BNNT synthesis, while MgO as catalyst, resulted in the formation of Mg2B2O5 which could not convert to BNNTs. In their experiment, a mixture of B, MgO, and FeO was heated up to a temperature of 1200 °C in an induction furnace to produce B2O2 vapor, and NH3 was introduced as the nitrogen source. BNNTs were produced at temperatures between 1100 and 1700 °C and have a diameter of ~ 50 nm, with small BN flakes as impurity. Variations of the BOCVD technique have also been demonstrated with differences in catalyst, experimental setup, precursor, and temperature [46, 63,64,65].
Another widely used BNNT synthesis technique is thermal CVD (TCVD), which involves the use of the growth vapor trapping (GVT). GVT makes use of an inner quartz tube with one end closed in a horizontal tube furnace. Lee et al. used a mixture of solid precursors (B, MgO, and FeO) in a 2:1:1 molar ratio placed near the closed end of the test tube at 1200 °C in the quartz chamber of an alumina combustion boat with ammonia gas to synthesize BNNTs. Continuous heating of the precursors for 1 h and reaction with NH3 produced white BNNT coatings. BNNTs were observed on the test tube, precursor materials, substrates, and around the inner walls of the alumina boat. The BNNTs produced had a diameter of 10–100 nm and length greater than 10 µm [59]. Other researchers such as Ferreira et al. [66], Pakdel et al. [67], and Ahmad et al. [68] have also used the TCVD technique, varying the precursor or catalyst.
Non-catalytic CVD
Producing BNNTs without the use of catalyst reduces one pathway for the introduction of impurities. Ma et al. synthesized BNNTs using CVD without the use of metal catalysts [69]. They utilized melamine di-borate (C3N6H6·2H3BO3) as a precursor to generate B–N–O compounds. The melamine di-borate was prepared by reacting melamine and boric acid in a hot aqueous solution in a molar ratio of 1:2, producing a white powder when cooled. The powder was later calcined and annealed in nitrogen gas to produce B4N3O2H as an intermediate product. The B4N3O2H intermediate was heated in an N2 atmosphere at 1700 °C to produce BNNTs. The BNNTs produced are well ordered having 12 concentric layers with a 5.2 nm and 13.1 nm inner and outer diameter, respectively. The BNNTs had bulbous tips made of a BN cage with encapsulated amorphous B–N–O clustered inside. In a later experiment, Ma et al. [70] adapted the previous technique, using microscale particles of α-Al2O3 as a substrate. The microscale alumina increased the inner diameter of the tube by 5 nm. The unusually large inner diameter produced via this method allowed the encapsulation of impurities within the tubes. The impurities included Si, Ca, amorphous B–N–O, and the formation of core filling crystalline boron carbide nanorods.
CVD is a versatile technique that has been used to synthesize BNNTs in a variety of configurations (Table 2). Each technique differs from the other in terms of precursors used, their ratios, substrate, the experimental setup and temperature, catalyst, and growth mechanism which resulted in a difference in quality, size, morphology, and quantity of produced BNNT. These variations also affect the type of impurities produced.
Whether BNNTs will be produced for research purposes or commercial and industrial applications, before choosing a synthesis method, it is important to understand the influence of the synthesis method on the quality of BNNTs produced. To assist the reader, Table 2 summarizes the discussed BNNT synthesis methods, highlighting the important synthesis properties, products, and impurities. Notably, this analysis shows that arc discharge, laser ablation, and a variety of CVD methods can produce high-quality BNNTs. However, the CVD technique has the highest potential to be refined for large-scale production of BNNTs because of its wide range of varying parameters. This can be achieved by selecting a CVD technique that can be optimized using readily available precursors and simpler reaction parameters (note: impurities/ byproducts that are easy to remove will be a bonus). Full analysis of synthesis methods is beyond the scope of this review. However, readers seeking a more detailed review of synthesis methods should look to Kim et al. [31], Ahmad et al. [56], Xu et al. [71], Yusuf et al. [72], Kalay et al. [73], and Tiano et al. [74].
Purification techniques
BNNT purification methods can be classified into physical and chemical techniques. The selection of purification method is highly dependent on the type/properties of impurities (as discussed in the previous section) present and the intended application of the nanotubes. The most challenging impurities to remove from BNNT products are those that are chemically and physically similar. As such, the removal of h-BN compared to other impurities (metal catalysts, amorphous boron/BN flakes, CNT-doped compounds, etc.) during the purification process is the most challenging because h-BN, as another BN allotrope, has similar chemical properties as BNNTs.
Sonication
Sonication is not a purification method when used alone. However, the importance of the role of sonication in many different purification processes merits inclusion here for discussion. Sonication is routinely used to disperse nanoparticles in liquids/solvents, loosen particles adhering to surfaces, and break up aggregates before or during purification techniques [75]. Sonication can be administered at various intensities. Bath sonication involves a transducer attached to a water bath serving as a secondary container. Cavitation and power are transferred from the bath through the sample container wall to the sample and are significantly gentler than tip-probe or cup-horn sonication [76]. Tip-probe and cup-horn sonication, often referred to as “ultrasonication,” involves a resonating probe or cup that is typically in direct contact with a sample dispersion. Cavitation and power transfer via ultrasonication are significantly more energetic [77]. Ultrasonication can create intense local pressures and temperatures in cavitation bubbles that can cleave chemical bonds, create radical species, catalyze reactions, and significantly damage nanoparticle structures [78, 79]. Sonication has been recognized as a useful process to assist purification processes to remove amorphous impurities from BNNTs in suitable solvents like water and ethanol when treated with ultrasound.
Xue-Song et al. [80] used ultrasonication to separate their “Yard-Glass” shaped BNNTs (YG-BNNTs), BNNTs with metal catalyst nanoparticles encaged in knob-like nodes, from graphitized filter paper substrate. The raw product containing the substrates and YG-BNNTs was ultrasonicated for 20 min in 200 mL ethanol, making it easier to peel the YG-BNNTs from the substrate. The YG-BNNTs, suspended in the ethanol, were filtered out but contained some fragments of the substrate. The ultrasonication did not completely separate the YG-BNNTs from the substrate, as there were substrate fragments left in the purified YG-BNNTs. Additionally, the ultrasonication process caused the YG-BNNTs to be broken into short fragments, demonstrating the potential negative impact of ultrasonication.
Chen et al. [29] also used the ultrasonic treatment to disperse large aggregates (up to several microns in size) of BN nanoparticles and nanotubes in ethanol. The result was the breakup of some large aggregates into fine nanotubes and nanoparticles and settlement of large aggregate and heavy metals at the bottom of the beaker after sedimentation of 20 min. This process left the top solution holding fine particles and nanotubes which were obtained by vaporizing the solvent [29].
Generally, the selection of bath or ultrasonication is mostly based on the nature of the material being worked on or the medium of dispersion. For example, bath sonication is usually employed when working with biological material like DNA [81], to prevent damage. Bath sonication is also used when dispersing in acids [82] to prevent the etching of the ultrasonicator probe tip.
Physical techniques
Methods dependent upon the physical properties of the tubes, such as solubility, size/morphology, or density of BNNTs do not require a chemical reaction, and therefore do not induce permanent modification of the BNNT surface. These methods include the use of surfactants, polymer wrapping, and filtration techniques. Physical purification techniques are desirable because these methods are mild and do not damage or chemically modify the tube structure. Sonication is often used with physical techniques; however, it is important to observe and fine tune experimental parameters (sonication time and power) to prevent excessive damage to the nanotubes.
Surfactant
Surfactants are used to preferentially solubilize either BNNTs or any of the impurities present in the sample before separation by filtration and/or centrifugation. A surfactant solution is formed with a dispersion of the BNNT sample. It is an amphiphilic solution containing one or more surfactants. In BNNT solubilizing solutions, surfactants and the solution medium are selected so that the surfactant preferentially assembles around the BNNTs, stabilizing them in solution. In a polar medium, such as aqueous dispersion, the hydrophobic part of the surfactant interacts with the BNNTs, while the hydrophilic part interacts with the water molecules enabling the dispersion of the BNNTs. The choice of surfactant should be able to provide adequate wetting and stable dispersion of the nanotubes and prevent aggregation of the tubes, and ideally, the surfactant should be easily removed for practical utilization of the nanotube properties.
Smith McWilliams et al. [27] studied the stability and quality of dispersion of BNNTs using aqueous solutions of eight surfactants. They found that nonionic surfactants with high molecular weight dispersed BNNTs more effectively, producing individualized tubes at high concentrations. However, large nonionic surfactants tended to be nonspecific stabilizers, while ionic surfactants like SDS, CTAB, and DTAB, were more specific in their dispersion of BNNTs. The study notes that a large number of h-BN were also found in the purified product [27], highlighting the unique challenge h-BN impurities pose during purification.
Triton X-100TM, a nonionic surfactant, was also studied by Vieira et al. [83] to create suspensions of as-grown BNNTs in distilled water [84, 85]. The suspension was bath sonicated and filtered through a Fluoropore FSLW (3 µm, 47 mm diameter) membrane to remove large clusters of h-BN and Ni catalyst impurities. The surfactant was removed from the purified BNNTs by washing with excess water and with further sonication before filtering through a Whatman PTFE filter (1 µm, 47 mm diameter) to remove smaller nanoparticles impurity aggregated with the tubes. Table 3 shows the list of some surfactants and their uniqueness (like ionic [86], polymerizable [87], and fluorescent [88]) used for aqueous dispersion of BNNTs.
Filtration
Filtration involves the use of a membrane with micro- or nanopores, where the paths may be straight or tortuous, to separate BNNTs from impurities based partly on size distribution. It also fractionates BNNTs based on length. It is useful for an application that may need a uniform diameter and/or length of BNNTs. Lee et al. [25] used stainless steel woven wire (pore size of 35 µm) as a porous membrane to separate BNNTs from h-BN impurities based on size and aspect ratio variation. The h-BN nanoparticles diffuse through the membrane while the BNNTs were retained on the membrane due to their higher aspect ratio. Vieira et al. [83] also conducted filtration using two membranes with different pore diameters. They used filter type Fluoropore FSLW (3 µm, 47 mm diameter) in the first step of filtration and Whatman PTFE filter type (1 µm, 47 mm diameter) in the second step. The results obtained showed that the larger pore size membranes retain all large aggregates whereas BNNTs were obtained using the smaller pore size membrane. One disadvantage of filtration noted is that some amorphous particles get stuck to the nanopores thereby reducing the effectiveness.
Gel column chromatography
Column chromatography is an excellent method for purifying and separating a mixture based on differences in specific properties or structure of its constituents. This technique employs a stationary phase composed of solid adsorbents such as silica gel and a mobile phase composed of liquid. By carefully designing the stationary phase and selecting different mobile phases, components in the mobile phase will travel at different rates, separating them into fractions. Recently, chromatography has been demonstrated for the purification of BNNTs.
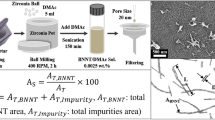
Ko et al. [89] used gel column chromatography to separate BNNTs from impurities while also separating them into length fractions. They accomplished this by utilizing a polymeric bead gel as the stationary phase and a variety of surfactants (sodium cholate (SC), SDBS, SDS, DOC, CTAB) to determine which were the most compatible with BNNT and could be used as the mobile phase. Sodium cholate provided a high nanotube dispersion efficiency with a high degree of nanotube individualization.
Figure 3 is the schematic of Ko et al.’s gel column chromatography setup used for BNNT purification and separation [89]. BNNTs were dispersed in 1 wt% SC aqueous solutions before being passed through the column. The sorting proposed here is based on adsorption effects and the size exclusion principle. Adsorption differences are determined by the strength of the interaction between BNNTs, h-BN, and B2O3 with the gel matrix, while the size exclusion is thought to be primarily responsible for the length sorting of the BNNTs based on retention time differences. The process successfully separated BNNT from its impurities and into different sizes, with a reported removal efficiency of 99.9% with length fractionation [89].
Schematics of gel column chromatography for BNNT purification. Reprinted with permission from [89].
Polymer wrapping and functionalization
Polymer wrapping and functionalization involve the use of polymeric compounds, typically with some polar blocks or groups, to selectively solubilize BNNTs, leaving the impurities as insoluble precipitates. Polymers can range from synthetic polymers like polyethylene, to modified biopolymer like ethyl cellulose, to natural biopolymers such as DNA. The polymer wrapping and functionalization mechanism can be thought of as a surface modification of the BNNTs and is achieved by preferential adsorptions of polymer compounds around individual BNNTs via non-covalent interactions [90]. Polymer wrapping is an attractive purification technique because it preserves the intrinsic properties of BNNTs and is a strong stable interaction [91]. Usually, the polymeric dispersion is treated by filtration, centrifugation, precipitation, and/or a dialysis step leaving behind polymer-wrapped BNNTs. While this method can effectively separate BNNTs from difficult contaminants like h-BN, the wrapped polymer molecule may act as contaminate if not required for the intended BNNT application. However, if smartly designed, it can be used to improve the application of the nanotubes [92]. As an example of smart design, De los Reyes et al. used ethyl cellulose, a non-aromatic polymer, to wrap, isolate, and disperse BNNTs. Using this technique, they successfully separated BNNTs from impurities like h-BN, and BN cages that were difficult to remove without damaging the tubes. The ethyl cellulose also aided their intended application to disperse BNNTs in select organic solvents. The ethyl functional groups improved the dispersion of BNNTs in benzyl alcohol, ethanol, methanol, and tetrahydrofuran [93].
There is historical precedence for DNA purification of nanotubes, first demonstrated with carbon nanotubes by Zheng et al. [94]. Kode et al. [95] has recently used DNA to separate BNNT from its impurities. They prepared an aqueous dispersion of as-synthesized BNNTs using single-stranded DNA, followed by a membrane filtration approach to remove undispersed impurities from the aqueous dispersions. The interactions between BNNTs and single-stranded DNAs were theoretically determined to be DNA wrapping on the BNNT via van der Waals interaction, that is, stacking of DNA’s hydrophobic nucleotide base and the BNNT’s hydrophobic surface in the aqueous environment [81].
Choi et al. used three types of polymeric compounds, oxidized polyethylene, polyoxypropylenediamine, and amine-terminated polyethylene glycol, to purify BNNTs samples synthesized by ball milling [96]. This wrapping purification took advantage of changes in density. The wrapped BNNTs were diluted with solvent followed by consecutive centrifugation separations. During the process, differences in solubility and differences between the density of impurities and polymer-wrapped BNNTs in the solvent created a density gradient separation which has long been an effective method of purifying a variety of nanotube-like materials. Similarly, Zhi et al. [91, 97] used a conjugated polymer PmPV, poly[m-phenylenevinylene-co-(2,5-dioctoxyp-phenylenevinylene)], to solubilize BNNTs in chloroform for purification. Subsequently, the PmPV-BNNT obtained was heat-treated in the air at 700 °C to remove the PmPv wrapping.
These methods do raise the issue of removal of the polymer after processing. Lee et al. [90] also considered using acid to remove the polymer residue wrapped with BNNT during purification. Methanesulfonic acid (MSA) was used to dissolve the poly(4-vinylpyridine) (P4VP) polymer bound to BNNTs. This takes advantage of the fact that the pyridine in P4VP is a Lewis base, which forms ionic bonds with protons preferentially over the weak dipole interactions with boron in BNNTs. When MSA was added to the polymer-wrapped BNNT solution, the BNNTs immediately aggregated due to the removal of the wrapped polymer and were eventually separated through filtration.
Predominantly, most reported BNNT polymer wrapping purification schemes are developed for direct application in polymer composites without removal of the polymer wrapping. However, thermal oxidation of wrapped BNNTs is possible due to their high thermal and oxidative stability above 800 °C, highlighting a major benefit of BNNT processing versus that of CNTs which oxidize near 400 °C with other carbon-based materials.
Comparatively, physical BNNT purification methods have not been widely adopted versus chemical methods considering that conditions are milder and typically do not damage the BNNTs. However, the perception seems to be that physical methods lead to incomplete purification.
Chemical purification techniques
Chemical purification techniques involve converting impurities such as elemental boron, BN particles, and h-BN into other compounds that can be removed easily. Oxidation of the sample is done with concentrated acids, a strong oxidant, or oxidation by oxygen/other gases at regulated temperatures to remove impurities. This selective oxidation is based on the oxidation temperature and rate differences of elemental boron, BN particles, h-BN, and BNNTs. Chemical purification techniques seem to be the most desirable methods currently implemented because the perception is that these methods can efficiently remove impurities, including the difficulty to remove h-BN. However, chemical methods are much more difficult to precisely control. Typically, harsh acids and high temperatures lead to significant damage to the tube structure, so there will be a trade-off between purity and the pristine properties of BNNTs. The chemical purification process can be classified based on the phase in which the reaction occurs: liquid phase versus gas phase.
Liquid phase oxidation: acid reflux
Concentrated acid reflux has been used to dissolve boron impurities and metal particles (catalyst or ball milling debris) associated with BNNT synthesis. This process is often referred to as “leaching.” Leaching sometimes results in chemical surface modification by reacting with BNNT dangling bonds. The effectiveness of removing the metal particles is dependent on the duration of oxidation, temperature, and concentration of acid used. Purification of BNNTs with low acid concentration and temperature for a short period will result in incomplete removal of metal particles while using acid with high concentration and temperature for a prolonged period will result in destruction or shortening of BNNTs, by attacking the BNNT defect sites [29]. Numerous acid refluxing processes with different acids, concentrations, and duration have been reported.
Xue-Song et al. [80] leached BNNTs with concentrated HCl solution (6 M) for 15 h at 70 °C to remove Fe-containing impurities. The HCl solution changes to a green color indicating the presence of Fe, after the reaction the BNNTs are removed via washing and filtration. Chen et al. [29] leached their BNNT samples using a 3 M HCl solution for 3 h at 90 °C to remove both iron and chromium metal catalysts. The products, FeCl3 and CrCl3, formed during acid leaching are soluble in hot water and therefore easily washed and filtered. The leached BNNT sample was dried at 100 °C and analyzed under EDS which showed a reduction in Fe content from 4.5 to < 0.1 wt% and Cr content from 0.7 to 0.2 wt%. Nitric acid treatment has also been used to remove boron nanoparticles by oxidizing them to water-soluble boric acid [74]. The nitric acid penetrates the BNNT shell and successfully removes the boron nanoparticles encapsulated, leaving behind a hollow BN shell. However, the BNNTs were found to be significantly damaged during the acid treatment.
Adnan et al. [98] purified BNNTs using solution processing with chlorosulfonic acid (CSA). From this work, we learn that this is a solubility-driven separation that takes advantage of BNNTs’ preferential solubility in CSA versus h-BN and other byproducts [99]. The as-synthesized BNNTs were mixed with CSA to form a gray slurry, which was then centrifuged to precipitate undissolved particles. The slurry supernatant was then washed with Chloroform to precipitate the BNNTs from the CSA. Following precipitation, ethyl alcohol and water are used to remove chloroform, hydrochloric acid, sulfuric acid, and other CSA-chloroform reaction byproducts. The recovered purified BNNTs were effectively separated from impurities which were found to be elemental boron, h-BN, and B2O3, with a small percentage of BNNTs present. Though this purification route was effective and does not change the chemical structure of the BNNTs, it is extremely important to note that CSA is a superacid and poses a significant hazard and is classified as potentially lethal [99].
The reaction of acids with BNNTs usually enhances the specific surface area. The specific surface area increase can imply several changes are occurring to the structure of the BNNTs. Surface area increases can result from the elimination of metal particles at the tube tips, the opening of tube ends, as well as the increase in defects and added chemical moieties to the BNNT tube walls.
Gas-phase oxidation: dry and wet thermal etching
Dry thermal etching involves the removal of B, BN, and metal impurities from the BNNT with selective high-temperature oxidation. The oxidation is done at a controlled temperature in an oxidative atmosphere to convert boron and BN impurities to forms of B2O3, which can be easily removed with hot water or ethanol washing. Also, chlorine gas can be used as an oxidative atmosphere to remove boron and BN impurities by converting them to BCl3 which is gaseous [100]. This technique is possible due to the high onset temperature of oxidation for BNNTs (~ 800 °C, higher resistance to oxidization) compared to the onset temperature for boron and amorphous BN impurities (~ 400 °C) in a similar size range.
Xue-Song et al. and Chen et al. took similar approaches, using high-temperature oxidation to remove BN and carbon impurities present in their YG-BNNTs [29, 80]. Thermogravimetric analysis (TGA) was conducted to establish an optimal oxidation temperature for removing fine BN impurities while preserving the BNNT structure/morphology. Fine BN nanoparticles are more reactive than BNNTs, therefore partial oxidation was established for removing the fine BN contaminants from the BNNTs without damaging the BNNTs themselves. Sharp weight loss was noted between 400 and 700 °C which corresponds to carbon oxidation, followed by a slow weight increase indicating oxidization of Fe-containing and BN impurities from 800 to 900 °C and rapid weight gain above 900 °C, related to oxidation of the BNNTs. The authors concluded the optimum temperature range for oxidizing BN (to solid B2O3) and carbon impurities was between 800 and 850 °C. The oxidized impurities were easily removed by hot water washing and filtration. Unfortunately, the presence of iron during the high-temperature oxidation created more difficulty to remove catalyst carbide nanoparticles (Fe3C) that remained encaged in the tubes. Ultimately, to remove the metal catalysts, HCl was used [80]. Throughout the literature, there seem to be slight disagreements when targeting an optimal temperature to oxidize BN impurities. Chen et al. [29] determined the optimal oxidation temperature for the effective removal of BN impurities in their sample (while the structure of the thin nanotube remains unchanged) to be 800 °C for 1 h. Harrison et al. [101] carried out the oxidation of their as-received BNNT at a temperature of 800 °C for 3 h to remove amorphous boron and BN impurities by forming boron oxide which was removed subsequently by hot water washing. Suggested temperatures range from 800 to 900 °C. While any temperature in this range will likely work, higher temperatures approaching 900 °C will increase the risk of oxidizing BNNTs. Differences observed by different group may be explained by operational differences like heating rate, heating time or more likely is a result of the presence of catalyst particles and/or due to structural differences between each groups synthesized BNNTs, including differences in diameter/chirality polydispersity, initial defect content, and number of walls.
Wet thermal etching is high-temperature oxidation done at a controlled temperature in an oxygen and steam environment. The B and BN impurities to be removed react with oxygen or water in the form of steam to form boron oxide, B2O3. Continuous exposure of boron oxide to steam results in the formation of meta-boric acid ([BOH]3O3) which is converted to aqueous boric acid (B(OH)3) and carried away as effluent exposing more surface for the reaction. In addition to boron oxide formation, BN particles form ammonia that is also carried out as effluent. This continuous reaction of oxygen, steam, boron, and BN increases the specific surface area and has been shown to react with edges of h-BN dangling bonds [30].
Wet thermal etching provides a solution to the problem of reduction in the exposed surface of impurities that may arise in dry thermal etching due to obstruction from solid boron oxide since wet thermal converts products to the gaseous phase to be carried off. Figure 4 represents the reaction conditions and wet thermal etching reactor. This general setup offers a possibility of being optimized to improve the yield of pure BNNT and can be scaled up to gram-level BNNT purification.
Schematic of wet thermal purification setup. (1 & 2) flow meters, (3) valve remains open, (4) Bypass valve- to provide an alternative path for nitrogen and dry oxygen to reach furnace, (5) Hot water source at 90 °C, (6) Heated stainless steel tube, (7) Tube furnace, (8) Empty flask, (9) Water, (10) Atmosphere. Adapted with permission from [30].
Gas/wet-phase thermal oxidation of amorphous boron and BN is advantageous versus acid treatment because the process can be more controlled. Typically, the thermal etching process preferentially oxidizes edges, ends, and existing defects in h-BN and BNNTs without introducing new tube wall defects. This should enable an application that requires BNNT alignment, unlike acid treatment that causes defects resulting in interactive clusters and increased aggregation of purified BNNTs.
Multi-step purification
The most efficient purification of BNNTs is accomplished by combining physical separation and chemical treatment in multi-step procedures. In most cases, multi-step processes are needed to remove metal particles, amorphous boron, or BN particles that were not sufficiently removed in a single treatment. Xue-Song et al. [80] used a combination of physical and chemical methods that involve ultrasonication, filtration, acid washing, high-temperature oxidation, and hot water washing to remove carbon, Fe, and BN impurities in the YG-BNNT sample. The ultrasonication removes most of the carbon substrate, the high-temperature oxidation removes carbon and oxidizes BN and Fe to B2O3 and Fe2O3, hot water washing removes the boron oxide B2O3, and acid washing with HCl removes Fe2O3. It was found that a combination of the methods was effective in obtaining purified BNNTs [102].
Harrison et al. [101] also used a series of methods to purify commercially produced BNNT. The first step used involved high-temperature oxidation and removal of amorphous material. Oxidation of the amorphous boron and BN was carried out at a temperature range of 750–800 °C to form boron oxide which was removed subsequently by hot water washing. A combination of sonication and filtration method called sonication-assisted isovolumetric filtration (SAIF) was used to further purify the oxidized-washed BNNT still containing h-BN impurities. The OW-BNNTs were sonicated in a solution (50:50 vol% of DMF: acetone) above the filter membrane with a tip probe to disperse the BNNTs. The dispersion was intermittently sonicated during filtration to maintain h-BN/BNNT disaggregation. DMF-acetone mixture was simultaneously added to maintain the dispersion volume in the filter. The method ensured the retaining of BNNT in a filter based on its size distribution [101]. Table 4 summarizes the description, advantages, and disadvantages of each purification technique.
Most techniques offer a balance of advantages and trade-offs that need to be optimized. For example, sonication techniques compliment purification by dispersing and individualizing BNNTs but can result in the breaking of BNNT into shorter fragments. Additionally, surfactants and polymers significantly enhance solubility and dispersion quality and can also increase purification selectivity, however, when left attached to BNNTs may act as impurities if they are not compatible with the desired application. The chemical methods can also cause undesired modification and/or etching of BNNTs to produce derivatized and shorter fragments. Optimal parameters for purification techniques will likely vary based on the composition of the as-synthesized BNNTs. Purification methods must align with the type of impurities present and the ultimate BNNT application (if known at the time of purification) as each technique has benefits and drawbacks. To determine these optimal parameters (temperature, concentration, exposure time, etc.), a reliable method of quantifying purity is needed.
Qualification and quantification of purification
Analysis and quantification of purity are an indispensable consideration when designing, optimizing, and selecting purification methods. BNNTs and h-BN possess nearly identical structural and chemical properties. Because h-BN is often the most abundant impurity produced during BNNT synthesis, it has been challenging to devise quantitative methods to measure BNNT content and purity. Various methods are being used to make a detailed characterization and description of as-synthesized samples and purified samples at different stages of the purification process. However, most analysis methods are qualitative or rely heavily on subjective quantification. Common BNNT analysis methods include transmission electron microscopy (TEM), scanning electron microscopy (SEM) [30], X-ray photoelectron spectroscopy (XPS), X-ray powder diffraction (XRD) [103, 104], Raman spectroscopy [105], Fourier-transform infrared spectroscopy (FTIR) [101], and thermalgravimetric analysis (TGA) [93]. These methods are used to determine the quantity and type of impurities present and for purity comparison.
X-ray photoelectron spectroscopy (XPS) is used to assess the quality of BNNT purification by analyzing and tracking changes in the elemental composition of raw BNNT samples and samples from different stages of its purification [106]. The XPS analysis provides a metric indication in ratio to measure the BN content, removal/reduction of impurity element, introduction of new element/functionality, and modification of the BNNT material. TEM is by far the most prevalent analysis tool for BNNTs. TEM is a powerful tool that can provide great insight into the quality of individual tubes, give an accurate measure of dimensions, and when complimented with electron energy loss spectroscopy (EELS), can provide atomic composition ratios. However, for purity analysis, the method is quite subjective and often involves counting tubes vs perceived impurities with single TEM images. Zhi et al. [91] used TEM to show the effects of the multi-step purification technique (HNO3 acid washing, polymer wrapping, sonication, and centrifugation) used by comparing the TEM image of the as-synthesized sample and the purified sample. The TEM image (see Fig. 5) of the purified sample showed the depletion of the impurities, which includes the BN particles, flakes, and fibers. Figure 6 is a representative SEM image from Zhi et al. [91], used to verify the morphology of the as-synthesized and purified BNNT samples.
TEM images of (a) as-synthesized, and (b) purified BNNT samples. Adapted with permission from [91].
SEM images of (a) as-synthesized, and (b) purified BNNT samples. Adapted with permission from [91].
Harrison et al. [101] used FTIR absorbance spectroscopy as a tool to quantify the h-BN impurities in the BNNT sample by comparing the variation of FTIR peak ratio with respect to varying h-BN concentration. XRD analysis was also used as a complementary technique to distinguish between samples with varying degrees of intensity. A major challenge in determining BNNT purity using common techniques such as, XPS, FTIR, UV–vis absorption, and Raman spectroscopies is distinguishing between BNNTs and other BN impurities because their spectroscopic signatures are similar. Other methods such as SEM and TEM are quite subjective, are time-consuming, and rarely accurately represent the bulk material.
An interesting and creative adaptation of UV–vis characterization is currently being developed by Martinez Rubi et al. [107] and Jakubek et al. [108]. They are investigating the use of absorption spectroscopy of regiorandom poly(3-hexyl-thiophene) (rra-P3HT) aggregates on BNNTs dispersed in chloroform to assess the purity quality of BNNTs. This is due to strong rra-P3HT’s selective interaction with BNNTs, which is accompanied by conformational changes. When rra-P3HT self-assembles on BNNTs dispersed in chloroform, it undergoes conformational changes accompanied by distinct colorimetric and spectroscopic changes. A free rra-P3HT solution in chloroform appears yellow/orange, whereas a fully dispersed rra-P3HT/BNNT solution in chloroform appears magenta/purple. These colorimetric changes were not observed in the presence of hexagonal boron nitride. As a result, the colorimetric gradient in various rra-P3HT/BNNT dispersions indicates the purity state of each BNNT sample. Qualification of samples under purification is usually done after the application of the individual technique to determine how well it worked, damages caused, and quantity/type of impurities left in the sample.
Applications
As BNNT production increases and purity inevitably improves, BNNTs will become one of the most important nanomaterials of this century. BNNTs have a broad potential for practical application in nearly all areas due to their unique properties. The effective use of BNNTs in application areas like biomedical and composite is highly dependent on purity. For most nanomaterials, they must be well dispersed and stabilized in the media (aqueous/non-aqueous) or matrix needed for its application.
Mechanical reinforcement of nanocomposites
BNNTs have been used as fillers in polymeric composites with poly(vinyl butyral), poly(methyl methacrylate), poly(ethylene vinyl alcohol), or polystyrene as the matrix to improve their thermal conductivity [8]. Loading amounts have been demonstrated from the low < 1 wt% to as high as 75 wt% of BNNTs incorporated into polymers [74]. BNNTs are attractive for use in composite materials for space applications not only due to their strong mechanical structure and low density but also for their neutron and space radiation shielding capability [109]. Generally, to improve the properties of the composite, a higher fraction of BNNTs is added but this does not guarantee better performance due to possible agglomeration of the BNNT in the composite. Adequate dispersion and good interfacial interaction with the matrix must be ensured, to improve the effectiveness of BNNT in composite. This is possible with the functionalization and modification of BNNT to be used [110].
Biomedical applications
BNNTs are attractive for biomedical applications because of their relative inertness. They are chemically stable and compatible with biological environments. BNNTs can be made hydrophilic by covalent modification of their surface [111]. Various studies have shown the possible use of BNNTs for tissue engineering [112], drug delivery (nanocarriers) [113], cell stimulation, and nano-transducers (due to its’ piezoelectric property) [114]. BNNTs have also been used as reinforcement for biological polymers and composites with hydroxyapatite (HA) used for orthopedic implants. BNNTs were used as phase reinforcement and grain size refiner for HA by Lahiri et al. [115] to improve its tribological and mechanical properties. HA has poor wear resistance and fracture toughness. The addition of 4 wt% of BNNTs improved the wear resistance of HA by 75% and increased the fracture toughness, elastic modulus, and hardness of HA by 86%, 120%, and 129%, respectively. Lahiri et al. [115] also reinforced biodegradable polylactide-polycaprolactone copolymer with BNNTs for application as orthopedic scaffold. They also showed the interaction of BNNTs with macrophages and osteoblasts to be non-cytoxic. Due to the possible non-cytotoxic nature of BNNTs, they are being used for diagnostic and therapeutic applications [114, 116].
Other applications
BNNTs have a wide bandgap (~ 6 eV) making them electrical insulators. As such, they have been utilized in the development of nano-cables using semiconducting nanowires as an insulating protective shield/capsule [117]. BNNTs have strong hydrogen adsorption capacity due to the dipolar nature of the B–N bonds, so they are useful materials in hydrogen storage applications [118]. The dipolar nature of B–N bonds is also responsible for the change in solid-state and molecular electronic device properties and optical properties of other materials in the systems [119]. When combined with other nanomaterials, BNNT can be used to make sensor devices to detect properties like humidity [120] and clinical diagnostics [121].
BNNTs also can suppress thermal neutron radiation which has been demonstrated in applications as a solid-state neutron detector as the neutron sensing element [74, 122]. A neutron detector made with BNNTs has greater efficiency and sensitivity than any other semiconductor-based neutron detector due to the maximized kinetic energy used to increase the production of electron–hole pairs. BNNTs also have various aerospace applications (such as aerogels, spacesuits, spacecraft, etc.) that require excellent materials that can resist extreme conditions. We have no doubt that in the near future BNNTs will be some of the most important nanomaterial components introduced into advanced composites and systems as they become more accessible. Even now, the full extent of BNNT utility and applications is beyond the scope of this review, which cannot cover all the applications of BNNTs. Readers looking for a more detailed review of BNNT applications should consult these recommended citations [31, 71, 73, 110, 111, 123, 124].
Conclusion
Studies on the application of BNNTs in different fields ranging from composite materials to biomedicine are on the rise due to their unique properties. However, the production of uniform high-purity BNNTs in large quantities has not quite reached the maturity of similar materials like CNTs. Due to the lower-purity BNNT products available on the market, purification techniques and qualification/quantification of those techniques are a critical necessity. The impurities found in a BNNT sample can be broadly classified into boron compounds and metals. Different purification methods are being used to improve BNNT purity and uniformity for desired applications, but quantification of purity remains challenging and largely subjective. Some emerging quantification techniques offer some less subjective feedback upon which to optimize purification parameters [101, 103]. The choice of physical and/or chemical purification method to be used is dependent on the synthesis method, impurities present, and sometimes possible application. Overall, the physical method is used to remove impurities based on physical properties like density, morphology, length, and size. In contrast, chemical methods are ideal for removing metals and catalysts, amorphous boron, BN flakes, h-BN, and amorphous B–N–O compounds by the reaction.
The purification of BNNTs with physical or chemical methods alone will likely be ineffective. The mild conditions of physical methods do not remove impurities with similar chemical and physical properties as the BNNTs but have the advantage of not modifying the BNNTs chemically and sonication can assist in breaking down large particles and dispersing BNNTs. Chemical methods are most commonly used for attaining high-purity BNNTs as they are effective in removing metal catalyst particles, amorphous boron, and BN contaminants. However, chemical treatment often results in the tip opening of the BNNT tubes, and many chemical purification methods lead to the introduction of functional groups resulting in BNNTs with different specific properties. The introduction of functional defects/groups or chemical modification can be used to improve the interface of BNNTs for specific applications but can result in decreased properties [125]. Research on covalent functionalization and surface modification of BNNTs combined with new and improved purification techniques is accelerating. Outlooks on commercial BNNT synthesis are positive and purity is consistently improving, but until high-purity BNNTs can be produced without the presence of difficult contaminants like h-BN, purification techniques and the quantification of those techniques will be an important aspect of BNNT research.
Data availability
Not applicable.
Abbreviations
- BN:
-
Boron nitride
- h-BN:
-
Hexagonal boron nitride
- BNNT:
-
Boron nitride nanotube
- BOCVD:
-
Boron oxide chemical vapor deposition
- CNT:
-
Carbon nanotube
- CSA:
-
Chlorosulfonic acid
- CTAB:
-
Cetyltrimethylammonium bromide
- CTAC:
-
Cetyltrimethylammonium chloride
- CTVB:
-
Cetyltrimethylammonium 4-vinylbenzoate
- CVD:
-
Chemical vapor deposition
- DOC:
-
Sodium deoxycholate
- DTAB:
-
Dodecyltrimetylammonium bromide
- FTIR:
-
Fourier-transform infrared spectroscopy
- GVT:
-
Growth vapor trapping
- HA:
-
Hydroxyapatite
- HTP:
-
High-temperature pressure
- MSA:
-
Methanesulfonic acid
- MWBNNT:
-
Multi-walled boron nitride nanotube
- OW-BNNTs:
-
Oxidized washed boron nitride nanotube
- PmPV:
-
Poly[m-phenylenevinylene-co-(2,5-dioctoxyp-phenylenevinylene)]
- rra-P3HT:
-
Regiorandom poly(3-hexyl-thiophene) SDBS, SC, DOC
- SEM:
-
Scanning electron microscopy
- SDBS:
-
Sodium dodecylbenzene sulfonate
- SDS:
-
Sodium dodecyl sulfate
- SC:
-
Sodium cholate
- SWBNNT:
-
Single-walled boron nitride nanotube
- TCVD:
-
Thermal chemical vapor deposition
- TEM:
-
Transmission electron microscopy
- TGA:
-
Thermalgravimetric analysis
- UV–vis:
-
Ultraviolet visible absorption spectroscopy
- XPS:
-
X-ray photoelectron spectroscopy
- XRD:
-
X-ray powder diffraction
- YG-BNNT:
-
“Yard-Glass”-shaped boron nitride nanotube
References
K.S. Kim, M.J. Kim, C. Park, C.C. Fay, S.-H. Chu, C.T. Kingston, B. Simard, Scalable manufacturing of boron nitride nanotubes and their assemblies: a review. Semicond. Sci. Technol. 32(1), 013003 (2016)
N. Sai, Microscopic theory for nanotube piezoelectricity. Phys. Rev. B (2003). https://doi.org/10.1103/PhysRevB.68.241405
X. Blase, A. Rubio, S.G. Louie, M.L. Cohen, Stability and band gap constancy of boron nitride nanotubes. Europhys. Lett. (EPL) 28(5), 335–340 (1994)
Y. Chen, J. Zou, S.J. Campbell, G.L. Caer, Boron nitride nanotubes: pronounced resistance to oxidation. Appl. Phys. Lett. 84(13), 2430–2432 (2004)
R. Arenal, A.C. Ferrari, S. Reich, L. Wirtz, J.Y. Mevellec, S. Lefrant, A. Rubio, A. Loiseau, Raman spectroscopy of single-wall boron nitride nanotubes. Nano Lett. 6(8), 1812–1816 (2006)
S. Dolati, A. Fereidoon, K.R. Kashyzadeh, A comparison study between boron nitride nanotubes and carbon nanotubes. Int. J. Emerg. Technol. Adv. Eng. 2, 470 (2012)
H. Shin, J. Guan, M.Z. Zgierski, K.S. Kim, C.T. Kingston, B. Simard, Covalent functionalization of boron nitride nanotubes via reduction chemistry. ACS Nano 9(12), 12573–12582 (2015)
C. Zhi, Y. Bando, T. Terao, C. Tang, H. Kuwahara, D. Golberg, Towards thermoconductive, electrically insulating polymeric composites with boron nitride nanotubes as fillers. Adv. Funct. Mater. 19(12), 1857–1862 (2009)
C.H. Lee, J. Drelich, Y.K. Yap, Superhydrophobicity of boron nitride nanotubes grown on silicon substrates. Langmuir 25(9), 4853–4860 (2009)
M.L. Cohen, A. Zettl, The physics of boron nitride nanotubes. Phys. Today 63, 34 (2010)
K.S. Kim, C.T. Kingston, A. Hrdina, M.B. Jakubinek, J. Guan, M. Plunkett, B. Simard, Hydrogen-catalyzed, pilot-scale production of small-diameter boron nitride nanotubes and their macroscopic assemblies. ACS Nano 8(6), 6211–6220 (2014)
B. Fakrach, A. Rahmani, H. Chadli, K. Sbai, M. Bentaleb, J.L. Bantignies, J.L. Sauvajol, Infrared spectrum of single-walled boron nitride nanotubes. Phys. Rev. B 85(11), 115437 (2012)
J.H. Kang, G. Sauti, C. Park, V.I. Yamakov, K.E. Wise, S.E. Lowther, C.C. Fay, S.A. Thibeault, R.G. Bryant, Multifunctional electroactive nanocomposites based on piezoelectric boron nitride nanotubes. ACS Nano 9(12), 11942–11950 (2015)
S.M. Nakhmanson, A. Calzolari, V. Meunier, J. Bernholc, M. BuongiornoNardelli, Spontaneous polarization and piezoelectricity in boron nitride nanotubes. Phys. Rev. B 67(23), 235406 (2003)
A. Krishnan, E. Dujardin, T.W. Ebbesen, P.N. Yianilos, M.M.J. Treacy, Young’s modulus of single-walled nanotubes. Phys. Rev. B 58(20), 14013–14019 (1998)
A.E. Tanur, J. Wang, A.L. Reddy, D.N. Lamont, Y.K. Yap, G.C. Walker, Diameter-dependent bending modulus of individual multiwall boron nitride nanotubes. J. Phys. Chem. B 117(16), 4618–4625 (2013)
X. Xin, M. Barger, K.A. Roach, L. Bowers, A.B. Stefaniak, V. Kodali, E. Glassford, K.L. Dunn, K.H. Dunn, M. Wolfarth, S. Friend, S.S. Leonard, M. Kashon, D.W. Porter, A. Erdely, J.R. Roberts, Toxicity evaluation following pulmonary exposure to an as-manufactured dispersed boron nitride nanotube (BNNT) material in vivo. NanoImpact 19, 100235 (2020)
T.H. Ferreira, E.M.B. de Sousa, Chapter 6—applications and perspectives of boron nitride nanotubes in cancer therapy, in Boron Nitride Nanotubes in Nanomedicine. ed. by G. Ciofani, V. Mattoli (William Andrew Publishing, Boston, 2016), pp. 95–109
S. Chatterjee, M.J. Kim, D.N. Zakharov, S.M. Kim, E.A. Stach, B. Maruyama, L.G. Sneddon, Syntheses of boron nitride nanotubes from borazine and decaborane molecular precursors by catalytic chemical vapor deposition with a floating nickel catalyst. Chem. Mater. 24(15), 2872–2879 (2012)
L. Li, L.H. Li, Y. Chen, X.J. Dai, T. Xing, M. Petravic, X. Liu, Mechanically activated catalyst mixing for high-yield boron nitride nanotube growth. Nanoscale Res. Lett. 7(1), 417–417 (2012)
T.H. Seo, G.H. Lee, A.H. Park, H. Cho, J.-H. Kim, S. Chandramohan, S.-R. Jeon, S.G. Jang, M.J. Kim, E.-K. Suh, Boron nitride nanotubes as a heat sinking and stress-relaxation layer for high performance light-emitting diodes. Nanoscale 9(42), 16223–16231 (2017)
T. Terao, C. Zhi, Y. Bando, M. Mitome, C. Tang, D. Golberg, Alignment of boron nitride nanotubes in polymeric composite films for thermal conductivity improvement. J. Phys. Chem. C 114(10), 4340–4344 (2010)
M.M. Rahman, S. Mateti, Q. Cai, I. Sultana, Y. Fan, X. Wang, C. Hou, Y. Chen, High temperature and high rate lithium-ion batteries with boron nitride nanotubes coated polypropylene separators. Energy Storage Mater. 19, 352–359 (2019)
X. Zeng, J. Sun, Y. Yao, R. Sun, J.-B. Xu, C.-P. Wong, A Combination of boron nitride nanotubes and cellulose nanofibers for the preparation of a nanocomposite with high thermal conductivity. ACS Nano 11(5), 5167–5178 (2017)
S.H. Lee, M.J. Kim, S. Ahn, B. Koh, Purification of boron nitride nanotubes enhances biological application properties. Int. J. Mol. Sci. 21(4), 1529 (2020)
V.K. Kodali, J.R. Roberts, M. Shoeb, M.G. Wolfarth, L. Bishop, T. Eye, M. Barger, K.A. Roach, S. Friend, D. Schwegler-Berry, B.T. Chen, A. Stefaniak, K.C. Jordan, R.R. Whitney, D.W. Porter, A.D. Erdely, Acute in vitro and in vivo toxicity of a commercial grade boron nitride nanotube mixture. Nanotoxicology 11(8), 1040–1058 (2017)
A.D. Smith McWilliams, C.A. de los Reyes, L. Liberman, S. Ergülen, Y. Talmon, M. Pasquali, A.A. Martí, Surfactant-assisted individualization and dispersion of boron nitride nanotubes. Nanoscale Adv. 1, 1096–1103 (2019)
A. Ismail, P. Goh, J. Tee, S. Sanip, M. Aziz, A review of purification techniques for carbon nanotubes. Nano 03, 127–143 (2008)
H. Chen, Y. Chen, J. Yu, J.S. Williams, Purification of boron nitride nanotubes. Chem. Phys. Lett. 425(4–6), 315–319 (2006)
D.M. Marincel, M. Adnan, J. Ma, E.A. Bengio, M.A. Trafford, O. Kleinerman, D.V. Kosynkin, S.-H. Chu, C. Park, S.J.A. Hocker, C.C. Fay, S. Arepalli, A.A. Martí, Y. Talmon, M. Pasquali, Scalable purification of boron nitride nanotubes via wet thermal etching. Chem. Mater. 31(5), 1520–1527 (2019)
J.H. Kim, T.V. Pham, J.H. Hwang, C.S. Kim, M.J. Kim, Boron nitride nanotubes: synthesis and applications. Nano Convergence 5(1), 17 (2018)
N.G. Chopra, R.J. Luyken, K. Cherrey, V.H. Crespi, M.L. Cohen, S.G. Louie, A. Zettl, Boron nitride nanotubes. Science 269(5226), 966–967 (1995)
A. Loiseau, F. Willaime, N. Demoncy, G. Hug, H. Pascard, Boron nitride nanotubes with reduced numbers of layers synthesized by arc discharge. Phys. Rev. Lett. 76(25), 4737–4740 (1996)
J. Cumings, A. Zettl, Mass-production of boron nitride double-wall nanotubes and nanococoons. Chem. Phys. Lett. 316, 211–216 (2000)
Y.-W. Yeh, Y. Raitses, B.E. Koel, N. Yao, Stable synthesis of few-layered boron nitride nanotubes by anodic arc discharge. Sci. Rep. 7(1), 3075 (2017)
M.W. Smith, K.C. Jordan, C. Park, J.-W. Kim, P.T. Lillehei, R. Crooks, J.S. Harrison, Very long single- and few-walled boron nitride nanotubes via the pressurized vapor/condenser method. Nanotechnology 20(50), 505604 (2009)
J.H. Kim, H. Cho, T.V. Pham, J.H. Hwang, S. Ahn, S.G. Jang, H. Lee, C. Park, C.S. Kim, M.J. Kim, Dual growth mode of boron nitride nanotubes in high temperature pressure laser ablation. Sci. Rep. 9(1), 15674 (2019)
D. Golberg, Y. Bando, M. Eremets, K. Takemura, K. Kurashima, H. Yusa, Nanotubes in boron nitride laser heated at high pressure. Appl. Phys. Lett. 69(14), 2045–2047 (1996)
M. Cau, N. Dorval, B. Attal-Tretout, J.L. Cochon, B. Cao, L. Bresson, P. Jaffrennou, M. Silly, A. Loiseau, E.D. Obraztsova, Laser-based diagnostics applied to the study of BN nanotubes synthesis. J. Nanosci. Nanotechnol. 8(11), 6129–6140 (2008)
G. Zhou, Z. Zhang, Z. Bai, D. Yu, Catalyst effects on formation of boron nitride nano-tubules synthesized by laser ablation. Solid State Commun. 109, 555–559 (1999)
M.S. Chang, Y.G. Nam, S. Yang, K.T. Kim, J.H. Yu, Y.-J. Kim, J.W. Jeong, Synthesis of boron nitride nanotubes via inductively coupled thermal plasma process catalyzed by solid-state ammonium chloride. J. Powder Mater. 25(2), 120–125 (2018)
A. Fathalizadeh, T. Pham, W. Mickelson, A. Zettl, Scaled synthesis of boron nitride nanotubes, nanoribbons, and nanococoons using direct feedstock injection into an extended-pressure, inductively-coupled thermal plasma. Nano Lett. 14(8), 4881–4886 (2014)
Y. Chen, L.T. Chadderton, J.F. Gerald, J.S. Williams, A solid-state process for formation of boron nitride nanotubes. Appl. Phys. Lett. 74(20), 2960–2962 (1999)
Y. Li, J.E. Zhou, K. Zhao, S. Tung, E. Schneider, Synthesis of boron nitride nanotubes from boron oxide by ball milling and annealing process. Mater. Lett. 63, 1733–1736 (2009)
S.K. Singhal, A.K. Srivastava, R.P. Pant, S.K. Halder, B.P. Singh, A.K. Gupta, Synthesis of boron nitride nanotubes employing mechanothermal process and its characterization. J. Mater. Sci. 43(15), 5243–5250 (2008)
L. Li, X. Liu, L. Li, Y. Chen, High yield BNNTs synthesis by promotion effect of milling-assisted precursor. Microelectron. Eng. 110, 256–259 (2013)
F. Liu, X. Zhang, J. Cheng, J. Tu, F. Kong, W. Huang, C. Chen, Preparation of short carbon nanotubes by mechanical ball milling and their hydrogen adsorption behavior. Carbon 41(13), 2527–2532 (2003)
D. Golberg, Y. Bando, K. Kurashima, T. Sato, Ropes of BN multi-walled nanotubes. Solid State Commun. 116, 1–6 (2000)
W. Han, P. Redlich, F. Ernst, M. Rühle, Synthesizing boron nitride nanotubes filled with SiC nanowires by using carbon nanotubes as templates. Appl. Phys. Lett. 75(13), 1875–1877 (1999)
X.Z. Wang, Q. Wu, Z. Hu, Y. Chen, Template-directed synthesis of boron nitride nanotube arrays by microwave plasma chemical reaction. Electrochim. Acta 52(8), 2841–2844 (2007)
D. Golberg, W. Han, Y. Bando, L. Bourgeois, K. Kurashima, T. Sato, Fine structure of boron nitride nanotubes produced from carbon nanotubes by a substitution reaction. J. Appl. Phys. 86(4), 2364–2366 (1999)
R.Y. Tay, H. Li, S.H. Tsang, L. Jing, D. Tan, M. Wei, E.H.T. Teo, Facile synthesis of millimeter-scale vertically aligned boron nitride nanotube forests by template-assisted chemical vapor deposition. Chem. Mater. 27(20), 7156–7163 (2015)
E. Borowiak-Palen, T. Pichler, G.G. Fuentes, B. Bendjemil, X. Liu, A. Graff, G. Behr, R.J. Kalenczuk, M. Knupfer, J. Fink, Infrared response of multiwalled boron nitride nanotubes. Chem. Commun. 1, 82–83 (2003)
D. Golberg, Y. Bando, W. Han, K. Kurashima, T. Sato, Single-walled B-doped carbon, B/N-doped carbon and BN nanotubes synthesized from single-walled carbon nanotubes through a substitution reaction. Chem. Phys. Lett. 308(3), 337–342 (1999)
M. Bechelany, S. Bernard, A. Brioude, D. Cornu, P. Stadelmann, C. Charcosset, K. Fiaty, P. Miele, Synthesis of boron nitride nanotubes by a template-assisted polymer thermolysis process. J. Phys. Chem. C 111(36), 13378–13384 (2007)
P. Ahmad, M.U. Khandaker, Z.R. Khan, Y.M. Amin, Synthesis of boron nitride nanotubes via chemical vapour deposition: a comprehensive review. RSC Adv. 5(44), 35116–35137 (2015)
O.R. Lourie, C.R. Jones, B.M. Bartlett, P.C. Gibbons, R.S. Ruoff, W.E. Buhro, CVD growth of boron nitride nanotubes. Chem. Mater. 12(7), 1808–1810 (2000)
C.C. Tang, M. Chapelle, P. Li, Y. Liu, H.Y. Dang, S. Fan, Catalytic growth of nanotube and nanobamboo structures of boron nitride. Chem. Phys. Lett. 342, 492–496 (2001)
C.H. Lee, J. Wang, V.K. Kayatsha, J.Y. Huang, Y.K. Yap, Effective growth of boron nitride nanotubes by thermal chemical vapor deposition. Nanotechnology 19(45), 455605 (2008)
C. Tang, Y. Bando, T. Sato, Catalytic growth of boron nitride nanotubes. Chem. Phys. Lett. 362, 185–189 (2002)
C. Tang, Y. Bando, T. Sato, K. Kurashima, A novel precursor for synthesis of pure boron nitride nanotubes. Chem. Commun. 12, 1290–1291 (2002)
C. Zhi, Y. Bando, C. Tan, D. Golberg, Effective precursor for high yield synthesis of pure BN nanotubes. Solid State Commun. 135(1–2), 67–70 (2005)
Y. Huang, J. Lin, C. Tang, Y. Bando, C. Zhi, T. Zhai, B. Dierre, T. Sekiguchi, D. Golberg, Bulk synthesis, growth mechanism and properties of highly pure ultrafine boron nitride nanotubes with diameters of sub-10 nm. Nanotechnology 22(14), 145602 (2011)
D. Golberg, Y. Bando, C. Tang, C. Zhi, Functional boron nitride nanotubes. 2010 3rd International Nanoelectronics Conference (INEC), 2010, pp. 47–48. https://doi.org/10.1109/INEC.2010.5424445
J. Li, J. Li, Y. Yin, Y. Chen, X. Bi, Water-assisted chemical vapor deposition synthesis of boron nitride nanotubes and their photoluminescence property. Nanotechnology 24(36), 365605 (2013)
T. Ferreira, P. Silva, R. Santos, E. Sousa, A novel synthesis route to produce boron nitride nanotubes for bioapplications. J. Biomater. Nanobiotechnol. 2, 426–434 (2011)
A. Pakdel, C. Zhi, Y. Bando, T. Nakayama, D. Golberg, A comprehensive analysis of the CVD growth of boron nitride nanotubes. Nanotechnology 23(21), 215601 (2012)
P. Ahmad, M. Khandaker, Y. Amin, Synthesis of boron nitride nanotubes by argon supported thermal chemical vapor deposition. Physica E 67, 33–37 (2014)
R. Ma, Y. Bando, T. Sato, CVD synthesis of boron nitride nanotubes without metal catalysts. Chem. Phys. Lett. 337(1–3), 61–64 (2001)
R. Ma, Y. Bando, T. Sato, K. Kurashima, Thin boron nitride nanotubes with unusual large inner diameters. Chem. Phys. Lett. 350, 434–440 (2001)
T. Xu, K. Zhang, Q. Cai, N. Wang, L. Wu, Q. He, H. Wang, Y. Zhang, Y. Xie, Y. Yao, Y. Chen, Advances in synthesis and applications of boron nitride nanotubes: a review. Chem. Eng. J. 431, 134118 (2022)
M. Yusuf, A.S. Khan, A review of synthesis and applications of boron nitride nanotubes (BNNTs) with future prospects. Curr. Smart Mater. 5(2), 66–78 (2021)
S. Kalay, Z. Yilmaz, O. Sen, M. Emanet, E. Kazanc, M. Çulha, Synthesis of boron nitride nanotubes and their applications. Beilstein J. Nanotechnol. 6, 84–102 (2015)
A.L. Tiano, C. Park, J.W. Lee, H.H. Luong, L.J. Gibbons, S.-H. Chu, S. Applin, P. Gnoffo, S. Lowther, H.J. Kim, P.M. Danehy, J.A. Inman, S.B. Jones, J.H. Kang, G. Sauti, S.A. Thibeault, V. Yamakov, K.E. Wise, J. Su, C.C. Fay, Boron nitride nanotube: synthesis and applications, in SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, vol. 9060. (SPIE, 2014)
I. Kaur, L.-J. Ellis, I. Romer, R. Tantra, M. Carriere, S. Allard, M. Mayne-L’Hermite, C. Minelli, W. Unger, A. Potthoff, S. Rades, E. Valsami-Jones, Dispersion of nanomaterials in aqueous media: towards protocol optimization. J. Vis. Exp. 130, 56074 (2017)
J. Jiang, G. Oberdörster, P. Biswas, Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 11(1), 77–89 (2009)
T. Meißner, K. Oelschlägel, A. Potthoff, Dispersion of nanomaterials used in toxicological studies: a comparison of sonication approaches demonstrated on TiO2 P25. J. Nanopart. Res. 16(2), 2228 (2014)
D. Kim, S. Nakajima, T. Sawada, M. Iwasaki, S. Kawauchi, C. Zhi, Y. Bando, D. Golberg, T. Serizawa, Sonication-assisted alcoholysis of boron nitride nanotubes for their sidewalls chemical peeling. Chem. Commun. 51(33), 7104–7107 (2015)
A.L. Tiano, L. Gibbons, M. Tsui, S.I. Applin, R. Silva, C. Park, C.C. Fay, Thermodynamic approach to boron nitride nanotube solubility and dispersion. Nanoscale 8(7), 4348–4359 (2016)
L. Xue-Song, C. Dian-Qiang, Z. Bo, Y. Jian-Lin, Purification of yard-glass shaped boron nitride nanotubes. Iran. J. Chem. Chem. Eng. (IJCCE) 33(1), 29–36 (2014)
V.R. Kode, K.R. Hinkle, G. Ao, Interaction of DNA-complexed boron nitride nanotubes and cosolvents impacts dispersion and length characteristics. Langmuir 37(37), 10934–10944 (2021)
D. Kim, H. Muramatsu, Y.A. Kim, Hydrolytic unzipping of boron nitride nanotubes in nitric acid. Nanoscale Res. Lett. 12(1), 94–94 (2017)
S.M. Vieira, D.L. Carroll, Purification of boron nitride multiwalled nanotubes. J. Nanosci. Nanotechnol. 7(9), 3318–3322 (2007)
S.M. Fatemi, M. Foroutan, Study of dispersion of boron nitride nanotubes by triton X-100 surfactant using molecular dynamics simulations. J. Theor. Comput. Chem. 13(07), 1450063 (2014)
H.S. Shamsuddin, P. Estellé, J. Navas, N. Mohd-Ghazali, M. Mohamad, Effects of surfactant and nanofluid on the performance and optimization of a microchannel heat sink. Int. J. Heat Mass Transf. 175, 121336 (2021)
J. Yu, Y. Chen, B.M. Cheng, Dispersion of boron nitride nanotubes in aqueous solution with the help of ionic surfactants. Solid State Commun. 149(19), 763–766 (2009)
S.H. Kang, S.W. Jeon, S.Y. Moon, Y.J. Yoon, T.H. Kim, Fabrication of noncovalently functionalized boron nitride nanotubes with high stability and water-redispersibility. J. Phys. Chem. Lett. 11(11), 4511–4516 (2020)
A.D. McWilliams, S. Ergülen, M.M. Ogle, A. Carlos, M. Pasquali, A.A. Martí, Fluorescent surfactants from common dyes—rhodamine B and eosin Y. Pure Appl. Chem. 92(2), 265–274 (2020)
J. Ko, H.M. Kim, S.Y. Moon, S. Ahn, S.G. Im, Y. Joo, Highly pure, length-sorted boron nitride nanotubes by gel column chromatography. Chem. Mater. 33(12), 4723–4732 (2021)
S.-H. Lee, M. Kang, H. Lim, S.Y. Moon, M.J. Kim, S.G. Jang, H.J. Lee, H. Cho, S. Ahn, Purification of boron nitride nanotubes by functionalization and removal of poly(4-vinylpyridine). Appl. Surf. Sci. 555, 149722 (2021)
C. Zhi, Y. Bando, C. Tang, S. Honda, K. Sato, H. Kuwahara, D. Golberg, Purification of boron nitride nanotubes through polymer wrapping. J. Phys. Chem. B 110(4), 1525–1528 (2006)
T. Fujigaya, N. Nakashima, Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci. Technol. Adv. Mater. 16(2), 024802 (2015)
F.D. De los Reyes, T. Fujieda, A. Takeuchi, T. Kawai, Y. Nonoguchi, Isolation of exfoliated boron nitride nanotubes via ethyl cellulose wrapping. Nano Sel. 2(8), 1517–1524 (2021)
M. Zheng, A. Jagota, E.D. Semke, B.A. Diner, R.S. McLean, S.R. Lustig, R.E. Richardson, N.G. Tassi, DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2(5), 338–342 (2003)
V.R. Kode, M.E. Thompson, C. McDonald, J. Weicherding, T.D. Dobrila, P.S. Fodor, C.L. Wirth, G. Ao, Purification and assembly of DNA-stabilized boron nitride nanotubes into aligned films. ACS Appl. Nano Mater. 2(4), 2099–2105 (2019)
J.-H. Choi, J. Kim, D. Seo, Y.-S. Seo, Purification of boron nitride nanotubes via polymer wrapping. Mater. Res. Bull. 48(3), 1197–1203 (2013)
C. Zhi, Y. Bando, C. Tang, R. Xie, T. Sekiguchi, D. Golberg, Perfectly dissolved boron nitride nanotubes due to polymer wrapping. J. Am. Chem. Soc. 127(46), 15996–15997 (2005)
M. Adnan, D.M. Marincel, O. Kleinerman, S.-H. Chu, C. Park, S.J.A. Hocker, C. Fay, S. Arepalli, Y. Talmon, M. Pasquali, Extraction of boron nitride nanotubes and fabrication of macroscopic articles using chlorosulfonic acid. Nano Lett. 18(3), 1615–1619 (2018)
O. Kleinerman, M. Adnan, D.M. Marincel, A.W.K. Ma, E.A. Bengio, C. Park, S.-H. Chu, M. Pasquali, Y. Talmon, Dissolution and characterization of boron nitride nanotubes in superacid. Langmuir 33(50), 14340–14346 (2017)
H. Cho, S. Walker, M. Plunkett, D. Ruth, R. Iannitto, Y. Martinez Rubi, K.S. Kim, C.M. Homenick, A. Brinkmann, M. Couillard, S. Dénommée, J. Guan, M.B. Jakubinek, Z.J. Jakubek, C.T. Kingston, B. Simard, Scalable gas-phase purification of boron nitride nanotubes by selective chlorine etching. Chem. Mater. 32(9), 3911–3921 (2020)
H. Harrison, J.T. Lamb, K.S. Nowlin, A.J. Guenthner, K.B. Ghiassi, A.D. Kelkar, J.R. Alston, Quantification of hexagonal boron nitride impurities in boron nitride nanotubes via FTIR spectroscopy. Nanoscale Adv. 1(5), 1693–1701 (2019)
H. Cho, J.H. Kim, J.H. Hwang, C.S. Kim, S.G. Jang, C. Park, H. Lee, M.J. Kim, Single- and double-walled boron nitride nanotubes: controlled synthesis and application for water purification. Sci. Rep. 10(1), 7416 (2020)
M.S. Amin, T.E. Molin, C. Tampubolon, D.E. Kranbuehl, H.C. Schniepp, Boron nitride nanotube impurity detection and purity verification. Chem. Mater. 32(21), 9090–9097 (2020)
S. Kalay, Z. Yilmaz, M. Çulha, Synthesis of boron nitride nanotubes from unprocessed colemanite. Beilstein J. Nanotechnol. 4, 843–851 (2013)
M.S. Amin, B. Atwater, R.D. Pike, K.E. Williamson, D.E. Kranbuehl, H.C. Schniepp, High-purity boron nitride nanotubes via high-yield hydrocarbon solvent processing. Chem. Mater. 31(20), 8351–8357 (2019)
M.B. Jakubinek, K.S. Kim, C. Homenick, O. Kodra, S. Walker, B. Simard, Assessment of boron nitride nanotube materials using X-ray photoelectron spectroscopy. Can. J. Chem. 97(6), 457–464 (2019)
Y. Martinez Rubi, Z.J. Jakubek, M. Chen, S. Zou, B. Simard, Quality assessment of bulk boron nitride nanotubes for advancing research, commercial, and industrial applications. ACS Appl. Nano Mater. 2(4), 2054–2063 (2019)
Z.J. Jakubek, M. Chen, Y. Martinez Rubi, B. Simard, S. Zou, Conformational order in aggregated rra-P3HT as an indicator of quality of boron nitride nanotubes. J. Phys. Chem. Lett. 11(10), 4179–4185 (2020)
M. Ghazizadeh, J. Estevez, A. Kelkar, Boron nitride nanotubes for space radiation shielding. Int. J. Nano Stud. Technol. (2015). https://doi.org/10.19070/2167-8685-150007e
C.H. Lee, S. Bhandari, B. Tiwari, N. Yapici, D. Zhang, Y.K. Yap, Boron nitride nanotubes: recent advances in their synthesis, functionalization, and applications. Molecules 21(7), 922 (2016)
G.G. Genchi, G. Ciofani, Bioapplications of boron nitride nanotubes. Nanomedicine 10(22), 3315–3319 (2015)
S.V. Anandhan, U.M. Krishnan, Boron nitride nanotube scaffolds: emergence of a new era in regenerative medicine. Biomed. Mater. (2021). https://doi.org/10.1088/1748-605X/abf27d
M. Emanet, Ö. Şen, M. Çulha, Evaluation of boron nitride nanotubes and hexagonal boron nitrides as nanocarriers for cancer drugs. Nanomedicine 12(7), 797–810 (2017)
G. Ciofani, S. Danti, G.G. Genchi, B. Mazzolai, V. Mattoli, Boron nitride nanotubes: biocompatibility and potential spill-over in nanomedicine. Small 9(9–10), 1672–1685 (2013)
D. Lahiri, V. Singh, A.P. Benaduce, S. Seal, L. Kos, A. Agarwal, Boron nitride nanotube reinforced hydroxyapatite composite: mechanical and tribological performance and in-vitro biocompatibility to osteoblasts. J. Mech. Behav. Biomed. Mater. 4(1), 44–56 (2011)
W.M. Silva, H. Ribeiro, J.J. Taha-Tijerina, Potential production of theranostic boron nitride nanotubes ((64)Cu-BNNTs) radiolabeled by neutron capture. Nanomaterials (Basel, Switzerland) 11(11), 2907 (2021)
W. Mickelson, S. Aloni, W.Q. Han, J. Cumings, A. Zettl, Packing C60 in boron nitride nanotubes. Science 300(5618), 467–469 (2003)
S.S. Han, J.K. Kang, H.M. Lee, A.C.T. van Duin, W.A. Goddard III., Theoretical study on interaction of hydrogen with single-walled boron nitride nanotubes. II. Collision, storage, and adsorption. J. Chem. Phys. 123(11), 114704 (2005)
G. Mpourmpakis, G.E. Froudakis, Why boron nitride nanotubes are preferable to carbon nanotubes for hydrogen storage?: an ab initio theoretical study. Catal. Today 120(3), 341–345 (2007)
Y. Yu, H. Chen, Y. Liu, L.H. Li, Y. Chen, Humidity sensing properties of single Au-decorated boron nitride nanotubes. Electrochem. Commun. 30, 29–33 (2013)
A.L.M. Reddy, B.K. Gupta, T.N. Narayanan, A.A. Martí, P.M. Ajayan, G.C. Walker, Probing of Ni-encapsulated ferromagnetic boron nitride nanotubes by time-resolved and steady-state photoluminescence spectroscopy. J. Phys. Chem. C 116(23), 12803–12809 (2012)
G. Ciofani, V. Raffa, A. Menciassi, A. Cuschieri, Folate functionalized boron nitride nanotubes and their selective uptake by glioblastoma multiforme cells: implications for their use as boron carriers in clinical boron neutron capture therapy. Nanoscale Res. Lett. 4(2), 113–121 (2008)
E.A. Turhan, A.E. Pazarçeviren, Z. Evis, A. Tezcaner, Properties and applications of boron nitride nanotubes. Nanotechnology 33(24), 242001 (2022)
D. Zhang, S. Zhang, N. Yapici, R. Oakley, S. Sharma, V. Parashar, Y.K. Yap, Emerging applications of boron nitride nanotubes in energy harvesting, electronics, and biomedicine. ACS Omega 6(32), 20722–20728 (2021)
H.B. Harrison, J.R. Alston, Sonochemical functionalization of boron nitride nanomaterials. MRS Adv. 5(14–15), 709–716 (2020)
Acknowledgments
Not applicable.
Funding
This work was funded by the NC Space Grant, Sub-Award # 2015-1942-AT-06.
Author information
Authors and Affiliations
Contributions
AM, HH, and JRA were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maselugbo, A.O., Harrison, H.B. & Alston, J.R. Boron nitride nanotubes: A review of recent progress on purification methods and techniques. Journal of Materials Research 37, 4438–4458 (2022). https://doi.org/10.1557/s43578-022-00672-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-022-00672-5